
How DECARBITE® Works
Removing Carbon Dioxide
The absorption of carbon dioxide removal or any acid gas using DECARBITE® is a chemical reaction, not a physical one. Carbon dioxide reacts with the sodium hydroxide based absorbent and undergoes a complete chemical change. This change is irreversible; therefore the absorbent cannot be regenerated for reuse.
A small percentage of moisture present in the absorbent material (about 3%) is important. CO2 reacts with this moisture to form carbonic acid, which in turn reacts with the hydroxide to form the salt of carbonic acid, or sodium carbonate .The absorption of carbon dioxide is expressed as follows:
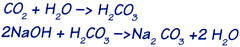
The products of reaction are sodium carbonate and water. DECARBITE® is disposable absorbent product.
DECARBITE® is color indicating, changing from tan to white upon carbon dioxide saturation. This change is clearly perceptible and indicates when spent material is to be discarded.
Occasionally, a condition known as channeling can occur when the gas flow finds holes or areas of least resistance. The gas flow follows these channels through the absorbent defeating the purpose of scrubbing out the carbon dioxide. DECARBITE® eliminates this problem in several ways; the silica binding to the sodium hydroxide keeps the particles from bonding in the presence of moisture which is formed as a by product of the absorption reaction. It also aids in preventing the absorbent to coalesce into a solid mass blocking gas flow and causing back pressure across the absorption bed.
Capacity
DECARBITE® absorbs more than 40% of its own weight under normal conditions.
System Design
Usually, transparent canisters or cylindrical tubes are used as this permits the visual inspection of the reaction during the removal of carbon dioxide from a gas stream. Such containers may be referred to as CO2 Getters, Co2 Scrubbers, CO2 Traps, CO2 Purifiers, CO2 Filters, Compress Gas Purifiers or CO2 Trap Cartridges. When glass tubes are used, they must be manufactured to resist high temperatures and corrosive effects.
Gas Flow
High gas flows impede the efficiency of the gas absorption by limiting the time of contact between the gas and the absorbent material. DECARBITE® granules have irregular surface areas and do not readily compact or fit together. The holding apart of the granules creates voids that allows for uniform gas flow and higher permeability. Gas flow resistance varies with particle size. Larger particle size ( 8-14 mesh range) allows greater space between particles and less resistance to gas flow (also smaller surface area). Smaller mesh size (14-30 mesh range ) have a larger over all surface area . The smaller particle size is the more efficient for fractional percentages of carbon dioxide absorption.
INDUSTRIES SERVED
Our products have served such industries as:- Analytical
Equipment
- Pure Gases
- Metallurgy
- Electronic Industry
- Research Institutions
- Manufacturing
Get around with Quick Links...
Home |
Carbon Dioxide Absorbent |
How Decarbite Works |
Decarbite Applications |
Quality Assurance |
Finished Product
Contact Us / RFQ |
SDS (PDF 110kb) |
Privacy Policy |
Site Map